JET8, as expert in handling life science-related products, not only provides transportation services but also support pharmaceutical companies and research institutions with storage services in our "GDP/GMP compliant pharmaceutical warehouse."
Feel free to contact us about GDP Warehouse.
JET8 GDP Warehouse - CryoMedia Pharma Archive -
Characteristics of Storage Service
GDP/GMP Compliant

We store your valuable pharmaceutical-related products in facilities that comply with the global standards of GDP/GMP for pharmaceutical and medical-related product storage.
Storage operations follow SOP (Standard Operating Procedures) in compliance with GDP/GMP standards
Licensed for pharmaceutical manufacturing (packaging, labeling, storage)
Storage of Hazardous Materials

Our facilities are authorized by the fire department to store flammable hazardous substances. This includes storage of Category 4 flammable liquids, ranging from 1 stone to 4 stones, such as alcohol-based liquids listed in the table below.
Hazardous Materials Category 4: Flammable Liquids
Toxic and Hazardous Substances
Highly Active Raw Materials
Genetically Modified Organisms

Our facilities can accommodate toxic and hazardous substances, highly active raw materials, and genetically modified organisms (excluding certain cases) in single tube units and beyond. You can also utilize our storage services for master storage of genetically modified cells and more.
*When entrusting us with these hazardous materials, we may require packaging in accordance with our company's regulations. We can also provide packaging materials if needed, so feel free to consult with us.
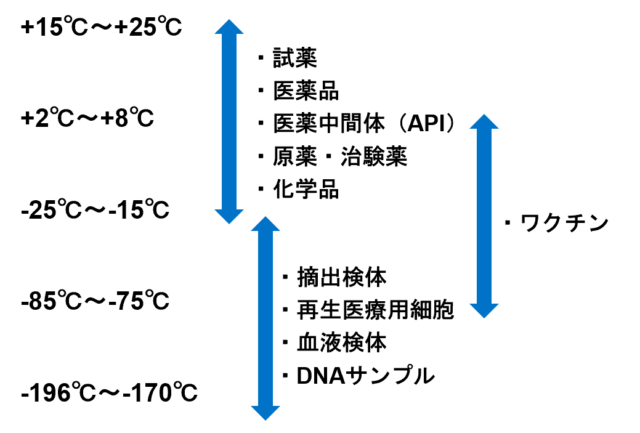
Temperature-Controlled Storage

We have dedicated temperature-controlled storage facilities equipped with 24-hour temperature monitoring capabilities. We offer storage options in 5 temperature zones tailored to your specific requirements.
Secure Zoning Management
Raw materials used for pharmaceutical intermediates, chemicals, regenerative medical cells, and more are stored in the Raw Material Storage Building, while samples and cells for archiving purposes are stored in the Sample and Cell Storage Building.
Emergency Preparedness
In case of a power outage at the storage facility, we have introduced the following equipment to maintain temperature control:
- Emergency cooling systems installed in each freezer and liquid nitrogen tank
- On-site 7-day operational emergency generator
- Adequate stock of dry ice and liquid nitrogen
Cargo Management System
To prevent mix-ups of your valuable cargo, we register all items with barcodes, serial numbers, and storage location addresses to keep track of cargo information.
End-to-end Handling
As experts in temperature-controlled biomedical transportation, we handle pick-up and delivery services before and after storage, both domestically and internationally.
- JET8 GDP Warehouse -
Tailored Solutions
We propose flexible storage solutions that align with your specific requirements, including cargo contents, storage temperature zones, cargo sizes, and duration.
Storage Periods
We can accommodate storage periods based on your requirements.
Cargo Sizes
We can handle cargo from a single tube unit.
- Microtubes
- Freeze boxes
- One-liter cans
- Petri dishes
- Various bottles/vials
- Drums
- Test tubes
- Cardboard boxes
- IBC containers

Storage Temperature Ranges and Acceptable Cargo
- JET8 GDP Warehouse -
Examples of Usage
Solution 1 Pharmaceutical Company - Supply Chain Division
Storage of imported Active Pharmaceutical Ingredients (API) in IBC containers at +5°C refrigeration.
Solution 3 Pharmaceutical Company - Research & Development Division
Storage of pharmaceutical samples for stability and accelerated testing in two temperature zones: refrigerated and room temperature.
Solution 4 Pharmaceutical Company - Production Division
Master storage of regenerative medical cells produced and sold by the company.
By utilizing Jet8's storage services, you can enjoy various benefits for your research, development, and production needs.
Business Continuity Planning (BCP) Measures
You can use our storage facilities as part of your "BCP measures" by distributing storage locations to different bases. This allows you to hedge against risks, as our storage facilities are established in suburban areas, protecting your valuable samples from unexpected risks such as disasters.
Capacity Expansion and Management Cost Reduction
Our storage services can supplement your facility's capacity if needed. Moreover, we can help reduce storage costs such as electricity and freezer expenses, and contribute to freeing up research time for researchers who previously dealt with complex storage management tasks.
We will provide services tailored to your needs.
Please feel free to contact us for further inquiries.
